Development of an Internet of things-based treatment adherence program among older adults with mild cognitive impairment using Intervention Mapping: A developmental study
Article information
Abstract
Purpose
Dementia and altered cognitive function are highly prevalent among older adults with mild cognitive impairment (MCI); hence, prevention is necessary before it develops into dementia. Treatment adherence—medication adherence and physical activity—is essential to prevent and delay dementia; however, comprehensive interventions to promote it in this population are lacking. This study aimed to develop a program for treatment adherence utilizing an Internet of Things (IoT) device.
Methods
The six-step mapping protocol was used to develop the IoT-based treatment adherence intervention (ITAI). The intervention was based on a literature review, expert opinions, and input from older adults with MCI.
Results
In Step 1, a needs assessment was conducted to gain insights into health problems and their underlying determinants. In Steps 2 and 3, performance objectives were identified for behavior change and selected theoretical and evidence-based methods were linked to the intervention outcomes. In Step 4, the ITAI was designed with components and materials consistent with the identified change goals and methods, and specific intervention components were developed. In Step 5, implementation plans and solutions to barriers to its application were identified. In Step 6, the plan to evaluate intervention effectiveness was outlined.
Conclusion
The Intervention Mapping provided a systematic procedure for developing an ITAI for older adults with MCI and preparing a randomized controlled trial. Utilizing Intervention Mapping is useful as ITAI systematically processes treatment adherence for MCI using the IoT and is acceptable and valid. ITAI is expected to increase medication adherence and physical activity in older adults with MCI.
INTRODUCTION
1. Background
Mild cognitive impairment (MCI) is a transitional stage between normal aging and dementia, characterized by subjective memory impairment, maintenance of normal cognitive function, and active daily living [1]. MCI increases the risk of dementia and cognitive function change, but it is reversible. Hence, prevention is necessary to avoid its development into dementia. The prevalence of MCI is increasing worldwide, and one out of five Korean older adults (20.2%) suffer from MCI [1]. The annual morbidity of dementia is 1%~2% and 10%~15% in normal older adults and those with MCI, respectively [2]. Hypertension, diabetes, hyperlipidemia, and atherosclerosis increase the risk of cognitive decline. Diabetes accelerates age-related cognitive decline [3], and increases the risk of dementia among older adults. Diabetes can also lead to complications in the central nervous system and cognitive function processes [3]. Additionally, decreased cerebral blood flow due to aging disrupts mechanisms regulating the brain and enhanced by hypertension. Furthermore, the severity of atherosclerosis in the arteries likely associates hypertension with cognitive decline [4]. People with MCI have a higher prevalence of hypertension, diabetes, and stroke [5]. According to the dementia prevention guidelines, the early detection of chronic diseases, treatment and management of chronic diseases, and regular exercise can prevent dementia [6]. Therefore, people with MCI need chronic disease treatment and regular exercise to reduce and prevent dementia. To manage chronic diseases, it is necessary to understand and follow experts’ instructions on drug use, diet, and exercise therapy [7]. Treatment adherence consists of taking medication and complying with a specialist’s prescription for chronic disease management [8]. Compliance with medication is essential for treatment adherence. Non-adherence to medication is a major barrier to safe and cost-effective health care delivery among providers. Medications and dosages are key factors in successful healthcare delivery, yet adherence in older adults remains low [9]. Cognitive decline in older adults is a risk factor for medication non-adherence [10]. It also affects instrumental daily activities, including medication use and treatment adherence [11]. For this reason, medication adherence intervention was implemented to solve the problem of medication non-adherence in older adults with MCI [12]. As with previous interventions, it helped people with MCI with medication adherence [12,13]. The mechanism of action associated with physical activity improves cognitive function, stimulates nerve growth, and encourages survival [14]. Physical activity decreases the risk of cerebrovascular diseases by reducing cardiovascular risk factors such as hypertension and hyperlipidemia [15]. Older adults with MCI have lower exercise levels than those with normal cognitive function [5]. A physical activity intervention study revealed that participants who adhered to medication reported improved cognitive function and decreased risk of cerebrovascular and cardiovascular diseases [16], suggesting that both regular physical activity and medication adherence are important in preventing cognitive decline.
The fourth industrial revolution brought in healthcare innovation through the development of information technology and biotechnology, with diseases being prevented using the Internet of things (IoT) platforms, and wearable devices and customized healthcare services showing significant growth [17]. The IoT indicates that each object connects to the Internet and enables communication through technology [17]. Research using the IoT has been conducted on individual interventions to provide educational information tailored to patients’ needs and technology-based reminders for patients and medical institutions [18]. Healthcare provider interventions are being developed based on IoT devices [19]. Notably, technology-based interventions improve patient outcomes, costs, and treatment effectiveness. Furthermore, technology-based interventions are employed for early recognition of inadequate performance; reducing complications; and alerting, rewarding, and providing feedback on the patient’s progress. A systematic review reports that regular visit strategies (telehealth, home monitoring, and telephonic counseling) have been widely employed to enhance medication adherence [20]. Interventions using periodic visits to improve medication are being challenged because sustained adherence could not be achieved. Considering that individuals with MCI experience memory deterioration, medication adherence intervention strategies are required to ensure continued use. For people with MCI, exercise not only prevents and delays dementia but also assists in managing chronic diseases [21]. However, a well-developed integrated approach for physical activity among older adults with MCI that ensures medication adherence, prevents and delays dementia and manages chronic diseases is unavailable. Technology-based exercises and physical activity rates record, and information and aid supervision are necessary strategies to support care managers’ and patients’ decisions when developing target programs.
The provision of healthcare through the convergence of the IoT and information and communication technology is recently adopted in managing chronic diseases because of their role in promoting treatment adherence through reminders. Smart health devices are developed and released to aid treatment methods for older adults with MCI [12,22]. IoT also allows immediate institutionalization of interventions by identifying the state and providing prompts for care and to medication adherence leading to desired treatment adherence, and these technologies can help patients with MCI adhere to treatment. This paper describes the methods used and outcomes obtained from developing and refining a theory- and evidence-based program to facilitate the IoT-based treatment adherence intervention (ITAI). The study aimed to develop an ITAI program to improve treatment adherence (medication adherence and physical activity) using Intervention Mapping steps (development of an intervention) among older adults with MCI.
METHODS
Ethics statement: The study was approved by the Institutional Review Board (IRB) of Yonsei University Health System prior to conducting the study (IRB No. 4-2019-1317).
1. Developing the ITAI
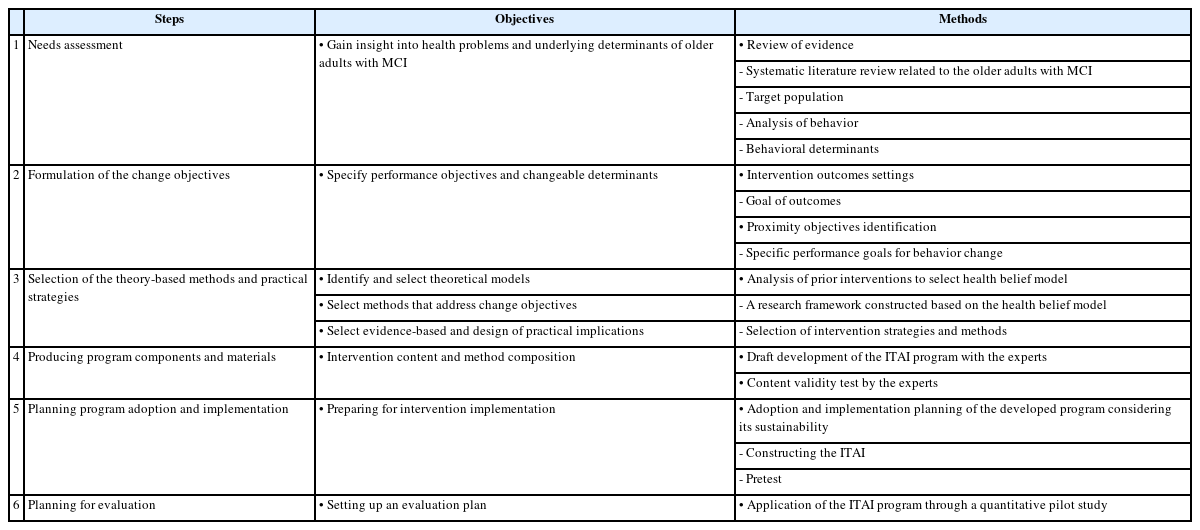
The development of the ITAI followed the Intervention Mapping protocol comprising six steps. The Intervention Mapping protocol is a systematic outline for the development, implementation, and evaluation of intervention for health behavior change [23]. It is considered useful for constructing programs grounded in both theory and empirical data. The Intervention Mapping facilitates the effective development of behavioral change interventions, and many healthcare programs have successfully used it for various interventions [24]. Table 1 shows the six steps of Intervention Mapping and their purpose and methods. The implementation as conducted in each step is presented below.
Step 1 required obtaining insight into the health problems and underlying determinants of older adults with MCI. We developed a complex intervention focused on behavioral support for MCI. Moreover, we first sought to understand the kind of support needed by an individual for their problem. Through this step, we gained insight into the health problems and underlying determinants of treatment adherence among older adults with MCI. To identify training and methods for providing them with the necessary support, we reviewed literature on the health problems associated with MCI. We searched PubMed and Cumulative Index to Nursing and Allied Health Literature (CINHAL) for English articles, and Korean studies Information Service System (KISS), KoreaMed, and Research Information Sharing Service (RISS) databases for Korean articles published until August 2020.
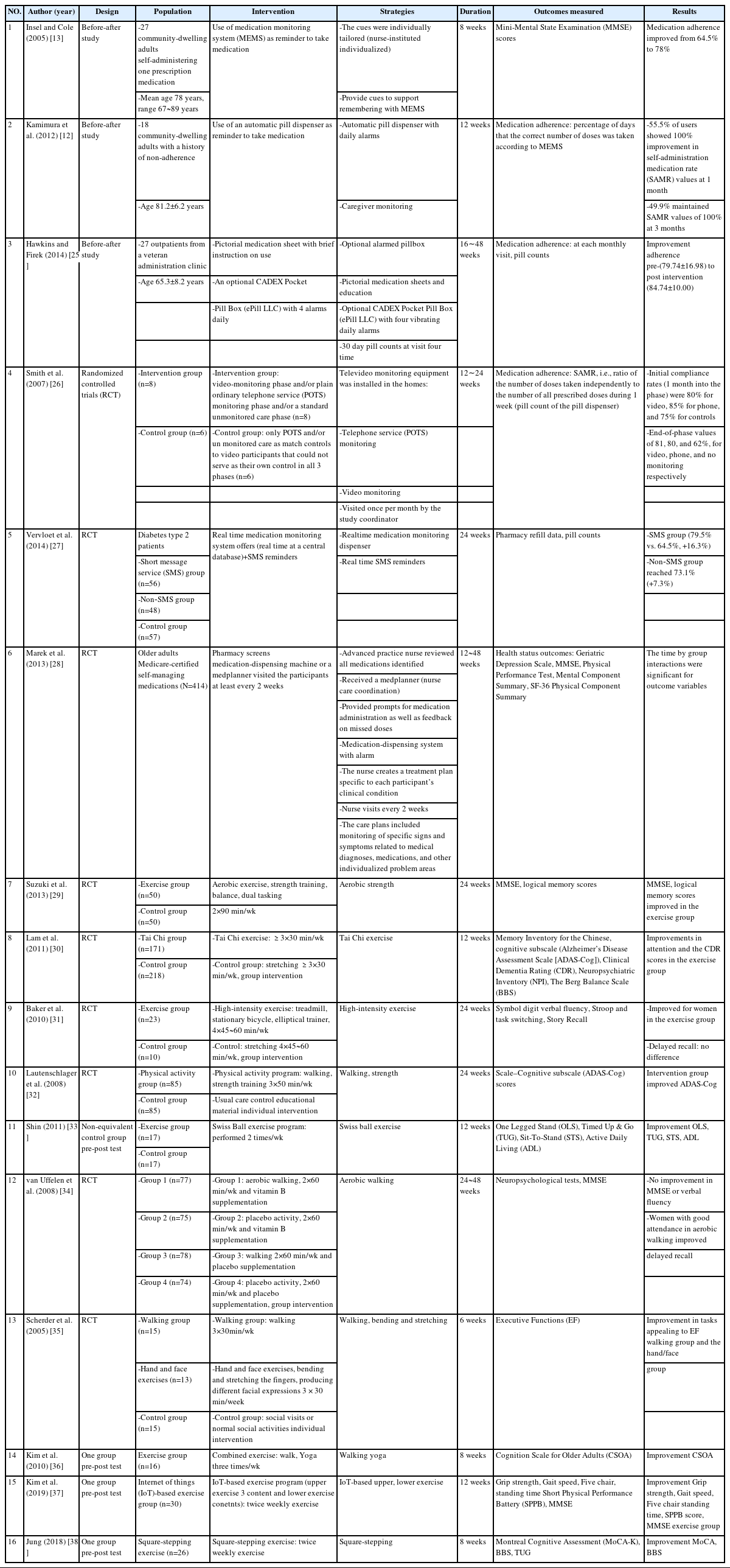
The literature review search terms were “mild cognitive impairment OR mild cognition impairment OR mild impairment of cognition OR mild impairment of cognitive function OR cognitive decline” AND “medication adherence OR medication compliance” AND “activity or activities OR walk OR walking OR exercise” AND “program OR programs OR intervention OR interventions OR treatment OR treatments.” The following criteria were used to select studies for analysis: (1) research published in English and Korea, (2) experimental or quasi-experimental studies, (3) research conducted for older adults with MCI, and (4) research with medication adherence and physical activity program. Finally, we analyzed 16 of 237 studies after excluding those irrelevant to the topic (Table 2, Appendix 1) [12,13,25-38].
In Step 2, we formulated the outcomes for health behavior change, dividing them into broad and specific performance objectives. We conducted a comprehensive literature review on medication adherence and physical activity among older adults with MCI in Step 1; based on this, overall behavioral goals and specific interventions for older adults with MCI were finalized.
In Step 3, a theoretical analysis was performed to identify effective health behaviors. A research framework was constructed based on the health belief model [39]. The performance strategy based on the health belief model was determined through the literature for health behavioral belief promotion education, self-efficacy improvement, cue to action trigger, cue to action trigger and reminder, and individualized coaching.
In Step 4, we designed the ITAI with components and materials consistent with the identified change goals and methods, described the components, and determined real-world applications. A discussion with six experts was conducted. Some of the experts had participated in the problem analysis in Step 1. To verify the validity of the expert group, the members were three nursing professors with expertise in health-related research and intervention program development, two gerontological nurse practitioners with more than 20 years of experience, and one neurologist.
In Step 5, after a week of pre-testing with participants with MCI, the research team met to discuss the implementation barriers and mitigation action plans.
In Step 6, we designed a randomized control trial pilot study to evaluate interventions. The program includes a nurse’s assessment of medication adherence and physical activity, weekly phone visits, and education. Major outcome measurement consists of items for treatment adherence; medication adherence, and physical activity level (gait phase and physical activity assessment) and additionally measures perceived health beliefs and self-efficacy.
2. Patient and Public Involvement
There are inadequately developed integrated approach for physical activity among older adults with MCI that ensures medication adherence, prevents and delays dementia and manages chronic diseases. This paper describes the methods used and outcomes obtained from developing and refining a theory- and evidence-based program to facilitate the ITAI. During the development process, the researcher was trained to conduct the intervention. The researcher participated in a one-day training course on the components of developing IoT interventions targeting older adults. The nurse involved in the intervention implementation received supervision and feedback from the first author, patients, and relatives on delivering the post-implementation interventions. Also, a trained nurse examined the pre-test of the intervention in five older adults with MCI from a neurology outpatient clinic who met the predetermined inclusion criteria. After ITAI was initially completed, five older adult patients with MCI tested the usability of the developed intervention. The patients and caregivers were informed that by signing this consent form, they consent to the collection and use of their information, and the data collected during the research will be used for research purposes. They were also informed that the findings would be published in international journals or through conferences without directly linking personal data to the collected information.
3. Delimitation of the Study
This study used Intervention Mapping to develop an ITAI for the management of MCI. Results from Steps 5 and 6 of the Intervention Mapping steps will be published in a future paper. We have applied only pre-testing using a feasibility test for the development of an intervention plan that can be implemented in future studies.
4. Ethics Approval and Consent to Participate
The study was approved by the IRB of Yonsei University Health System prior to conducting the study (IRB No. 4-2019-1317).
RESULTS
1. Step 1: Needs Assessment
In the first step of the needs assessment, we reviewed and analyzed the health problems and behaviors of MCI to identify the determinants. Furthermore, the final participation criteria were as follows: aged 65 years or older, diagnosed with MCI by a neurologist, no previous history of any psychiatric disease, taking medications for a chronic disease, using a smartphone, and those who have a primary caregiver. People with a diagnosis of dementia and severe respiratory and cardiovascular disease were excluded.
1) Analysis of Behavior
Compared to older adults in general, those with MCI suffered from more chronic diseases such as hypertension and diabetes [3]. Medication adherence among community-dwelling individuals with MCI was reportedly between 64.8% and 75.0% [13,26]. As most participants with MCI lived in their homes, the need for an integrated intervention to improve the adherence of doctor-prescribed medication and physical activity (hereafter referred to as treatment adherence) for dementia prevention was necessary.
We found that older adults with MCI perceived difficulties in performing healthy behaviors. Although they could perform normal activities, they had memory impairment due to cognitive decline, which means that even though they thought of implementing healthy behaviors, they could not practice them due to a decline in memory. Cognitive decline in such individuals, therefore, acts as a risk factor for lower medication adherence [40] and affects instrumental activities of daily living, rendering it difficult to comply with treatment [11]. Although exercise is recommended for those at risk or individuals who are living with dementia, many are inactive [41]. Older adults with MCI are less active than those without it, and few meet the recommended physical activity for maintaining their health and functioning [42]. The tendency for low physical activity and fitness in people with MCI or dementia may be due to different barriers concerning exercise participation. Because these people are generally older, they must fight the perceived age-related barriers to exercise participation, such as fear of falls, increased risk of injury, and a perception of limited benefits of exercise [42]. Education is needed to overcome these barriers. Moreover, it is essential to improve the perceived health beliefs related to physical activity [39].
2) Analysis of Behavioral Determinants
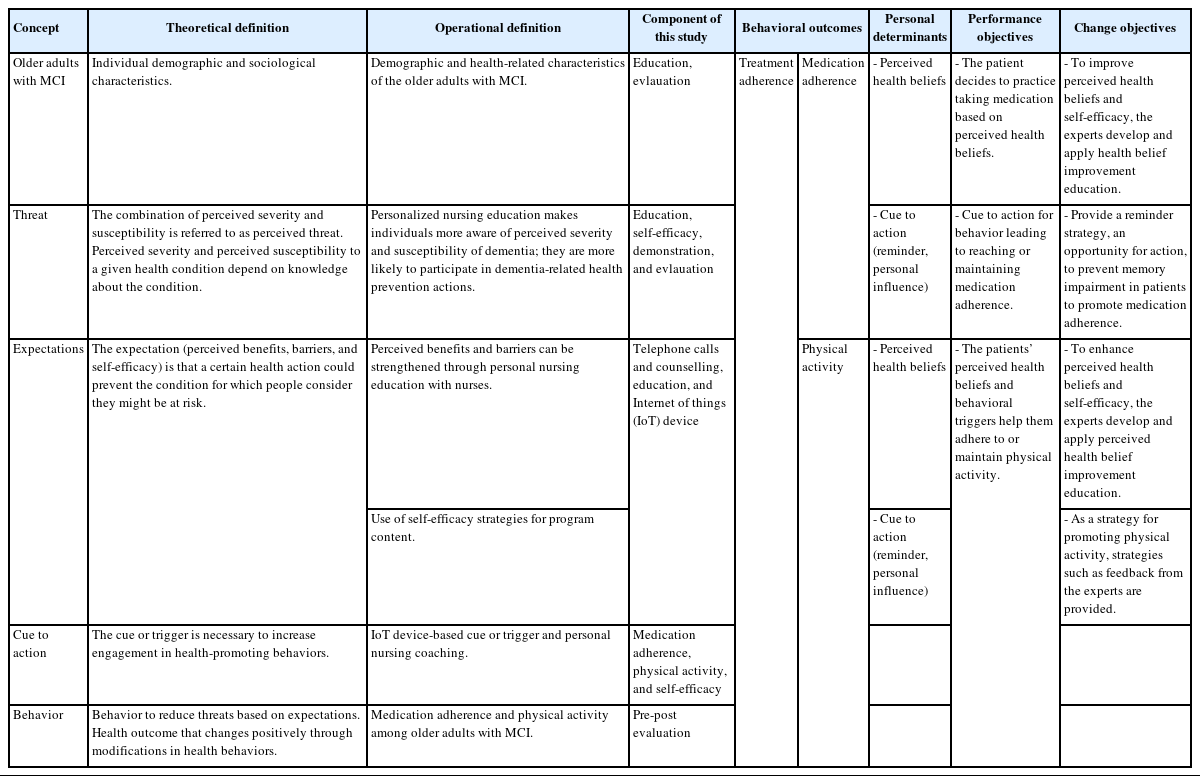
In this study, we confirmed the theoretical definition and developed an operational definition of treatment adherence. The former refers to the degree to which an individual performs behaviors consistent with a clinical prescription [8]. The latter entails the adherence to prescribed medication for chronic diseases and physical activity. We considered the major behavioral determinants of individuals with MCI based on the health belief model to identify the factors that could change behaviors for treatment adherence. The main factors affecting the health behavior of participants with MCI are problems of cognitive impairment, which do not allow behaviors to be practiced owing to the lack of perceived health beliefs (threats and expectations) [43] and memory deterioration. Exercise providers promote movement in these groups by recognizing and responding to the needs of people with MCI or dementia in exercise programs [41]. Furthermore, cue to action interventions have been implemented to help memory by focusing on strategies that trigger actions [13]. However, even if the perceived health beliefs are enhanced, it is difficult to practice them due to memory impairment. Specifically, we recognized the need for a method that could help improve health beliefs and memory for people with MCI to practice health behaviors.
2. Step 2: Outcomes and Objectives
The second step was to identify intervention outcomes based on needs assessment. The intervention goal was treatment adherence by improving the patients’ medication adherence and performance of physical activities, to reduce the risk of dementia. We defined behavioral outcomes as an “increase in treatment adherence.” As perceived health beliefs and behavioral triggers cause these treatment adherence behaviors, the performance goals should be devised around them. Subsequently, we established objectives by selecting important and modifiable determinants of behavior, taking medication based on perceived health beliefs and creating cues to action for behavior leading to reaching or maintaining medication adherence. To achieve these, we formulated the change goals (Table 3).
3. Step 3: Theoretical Methods and Practical Strategies
In the third stage, we identified and selected theoretical models and evidence-based methods to address the change objectives described earlier. The health belief model was found to be appropriate because it accounted for health behavior changes, including key motivational factors and individual perceptions, to explain preventive health behaviors. The health belief model is divided into risk and expectation factors in the perceived health beliefs; based on this classification, health behaviors can be practiced using a cue to action. Health beliefs refer to behaviors regarding disease control that help in behavior change and function as prerequisites for action [39]. The health belief model consists of five concepts of behavioral change: perceived severity, susceptibility, benefit, barriers, and self-efficacy [39]. To develop the interventions, we confirmed the theoretical definition of the health belief model, established an operational definition, identified the components of this study (Table 3), and constructed a framework.
1) Behavior Change Techniques
After identifying the patient and ITAI target behaviors, interventions were developed for older adults with MCI; an intervention method using the IoT to provide reminders was selected for action trigger strategies [25]. Additionally, perceived health beliefs promotion education and self-efficacy enhancement strategies were developed. Recently, devices that help medication adherence can prompt and monitor drugs based on the IoT, enabling the use of technology-oriented reminders [18]. As a strategy to promote physical activity, recommended walking (cardio exercise) that is not limited by time and space was selected as it could easily induce regular exercise in older adults [32]. Regarding the IoT, wrist-worn devices promoted walking. We further developed personalized goals and contents for their achievement as follows: the ITAI based on perceived health beliefs improvement education and the self-efficacy enhancement strategy; the IoT smart pillbox, wrist-worn devices, and action trigger strategies as reminders [25]; and personalized nursing coaching strategies [26].
4. Step 4: Developing Intervention Components
1) Development of the ITAI Content
The fourth stage involved designing an ITAI with components and materials consistent with the change goals and methods identified in Step 3, which was discussed with the expert group. The individual components and the real-world applications that were developed were described. First, design problems were identified by reviewing the literature on interventions based on the health belief model [39] and those using the IoT. Second, data on communication technology information about the device were obtained. Finally, educational programs on the perceived severity, susceptibility, benefit, and barriers (perceived health beliefs) were developed as a strategy for improving health behavior beliefs. The intervention strategy consisted of self-efficacy enhancement approaches, including achievement experience, proxy experience, and verbal persuasion [44]. To improve an individual’s perceived health beliefs, participants and their primary caregivers were educated on disease knowledge, dementia risk factors, the importance of medication adherence and physical activity, how to exercise walking, and how to practice drug use. Personalized training methods were implemented via handouts, and pre-intervention training was provided to participants lasting approximately 20~30 minutes. During the intervention period, for customized nursing coaching, an IoT-based smart pillbox alerted the user to take medicines, and a wrist wearable device reminded the user to go walking. In the introductory step, we trained a researcher (registered nurse) in prescription recommendations, IoT smart pillbox, and wrist wearables, using handouts, educational material, and demonstrations. Personalized nursing coaching, which is conducted via weekly telephone consultation, lasting about 20 minutes enquired about feedback on the patient’s medication adherence rates, the average weekly steps, and then the weekly steps goals for the ensuing week. Additionally, in real-time, the researcher immediately checked the medication adherence status through the data transmitted from the smart pillbox. If the patients failed to take medication more than twice, the researcher instantaneously contacted them via phone and sent a text message to the primary caregiver. Primary caregivers provide the most care for older adults with MCI, mainly daughters, sons, and daughters-in-law in Korea. When the primary caregivers received the text message, they were instructed to visit or call the older adults with MCI or help them take their medications. During the maintenance period, a reminder strategy was implemented, excluding customized nursing coaching, to maintain a reminder set for a personalized smart pillbox and a wearable alarm set as a maintenance strategy to promote physical activity.
2) Content Validation
The panel of five experts (comprising physician, nursing professors, and geriatric practitioners with at least 10 years of experience) evaluated the education and program content validity, and the relevance of the ITAI using the Polit and Beck methods [45]. We measured the content validity index of the overall ITAI program using the scale content validity index. The proportion of education and program content that reported a rating of 3 or 4 by all content experts (scale content validity index/universal agreement), the average of the item content validity index for all items on the scale (scale content validity index/average proportion), and the content validity index of ITAI individual content items (item content validity index) [46]. The scale content validity index is a composite score that requires key components like the scale content validity index/universal agreement, scale content validity index/average proportion, and the item content validity index. Each item was rated on a 4-point scale to avoid having an ambivalent midpoint: 1, not relevant; 2, somewhat relevant; 3, quite relevant; and 4, highly relevant. The experts’ scores are assigned relevance when they score 3 to 4 on the Likert-type scale. When the assigned score is deemed as relevant then a 1 is assigned and when it is deemed not to be relevant then a 0 is assigned. The individual item content validity index ratio was computed as the number of relevant ratings, therefore, dichotomizing the scale into either relevant (1) or not relevant (0). As a result, the scale content validity index/universal agreement, scale content validity index/average proportion, and scale content validity index/universal agreement ranged from 1.00. The criteria of the dimensions that indicated sound content validity were item content validity index >0.78 and scale content validity index/average proportion >0.90 (Appendix 2).
5. Step 5: Implementation Plan
The ITAI will be planned as a pre-post design with a control group, comprising an intervention period of 6 weeks, and a maintenance period of 4 weeks based on the 16 articles included in the literature review. We discussed the potential issues and barriers to intervention implementation and devised a plan to ensure the execution with the expert group. Additionally, the researcher was trained to conduct the intervention. The researcher who provided the intervention participated in a one-day training course on the components. Subsequently, the nurse received supervision and feedback on how they delivered the post-implementation interventions. A trained nurse examined the pre-test of the intervention in five older adults with MCI. After ITAI was initially completed, five older adults tested its usability. During the pre-test, the project leader supervised the nurse to ensure that the assessment was carried out as described. After a week of pre-testing, the five older adults with MCI and a research team met to discuss the barriers to implementation to develop an action plan to overcome the barriers. The main difficulty identified during the testing phase was that older adults with MCI forgot to use the device due to memory impairment. To solve this problem, we created a poster with an illustration and provided a description of the device, and placed it in the patients’ homes where they could observe it while taking medications.
6. Step 6: Evaluation Plan
The evaluation of the intervention is part of the implementation process. We conducted a pre-test and designed a randomized controlled trial to assess the feasibility of the intervention, and investigated the delivered practice. The goal of the intervention is to enhance medication adherence, physical activity, perceived health beliefs, and self-efficacy. The final IATI for older adults with MCI is presented in Table 4 and Figure 1. In the first week, which is the start of the intervention, the patients are given a demonstration of the IoT device in their homes. The nurse evaluates the physical activity assessment and medication adherence. Thereafter, the researcher provides face-to-face education for medication adherence and physical activity. The patients implement daily activity (steps count), which is monitored through a wearable device. For 1 to 6 weeks, the nurse researcher performs daily checks through real-time monitoring of medication adherence through the webserver. If medication non-adherence happens twice in a row, the nurse makes an immediate call to the participant and sends a text message to the caregiver. Furthermore, nurses provide weekly phone visits to provide feedback and motivation. For seven to 10 weeks, the participants themselves implement medication adherence and physical activity enhancement with the help of an IoT device. The primary outcomes are medication adherence rates and the average weekly step count. This study will have a duration of 10 weeks, and the outcome variables will be assessed at baseline, 6 weeks, and 10 weeks. Outcome assessment measures will include perceived health beliefs, self-efficacy, medication adherence, and physical activity levels. The outcomes will be analyzed using a linear mixed-effects model with random effects and repeated measures effects.
DISCUSSION
The use of Intervention Mapping ensured an efficient approach to intervention development, including the participation of the target population. It was ascertained that the intervention was systematically approached and based on the available evidence and theories. It was grounded in theory so that the project planner could specify the essential determinants and outcome factors. This framework also made it easy to determine what needed to be changed due to the intervention. In this study, we described the systematic evolution of the ITAI interventions according to the Intervention Mapping.
The goal of our intervention was to improve treatment adherence by enhancing medication adherence and physical activity so that dementia could be prevented or delayed in people with MCI in the long term. Previous interventions to promote medication adherence [10,18] and physical activity [32] exist, but relatively few interventions include a real-time approach and feedback. The program developed in this study is useful as it is an ITAI with a real-time approach focusing on treatment adherence and fully considers the environment in which the technology-based healthcare service influence has grown. This program is based on the theory of health belief behavior designed to increase treatment adherence and prevent cognitive decline in older adults with MCI. The ITAI will help improve treatment adherence outcomes, and ultimately, prevent or delay dementia and improve health status among community-dwelling older adults with MCI. Community health workers, healthcare providers, and administrators can easily access older adults with MCI living at home or in institutions and improve treatment performance.
The Intervention Mapping enabled us to describe and design our intervention using selected strategies that are essential for older adults with MCI. The Intervention Mapping was found to be a beneficial and systematic way to describe interventions; however, to ensure standardization using the IoT, we spent extensive time identifying and using IoT-based Korean products. This was done to select a product that would suit the patients’ individual needs. This paper explained the interventions in detail and with transparency to inspire other programs that are being developed using different methods.
Community-dwelling older adults with MCI have been observed to have varying impairments and considerable comorbidities. The ITAI is intended to provide support to older adults with MCI with some degree of independence who live in communities rather than those who receive institutional care or depend on domiciliary carers. Older adults with MCI within some communities may have considerable physical function limitations or debilities and may be depressed. Therefore, our intervention might not entirely meet their needs and treatment adherence. As a result, those with specific difficulties (e.g., comorbid chronic illnesses, physical function limitations, behavioral and psychological symptoms) may require some tailored goal adherence support from a nurse, doctor, or pharmacist.
The possibility of accepting IoT devices for older adults with MCI was confirmed. The ITAI in this study can be managed at the patient’s home in real-time, and the health manager can check the patient’s treatment progress anytime, anywhere, making immediate intervention possible, reducing cost and time, and increasing the effect. However, older adults with MCI needed several educational sessions and demonstrations as one or two sessions were insufficient. The nurse researcher had to visit the participants’ homes several times because they forgot to use the device due to a malfunction or memory loss. Thus, the extent to which the provider intervention contributed to increased treatment adherence rates is unclear. For this reason, during the intervention period, older adults with MCI were asked to identify additional barriers by identifying the number of training and demonstration sessions required. Provider training was designed for providers serving older adults with MCI and might not be effective in diverse settings or contexts as the materials used may not resonate with other populations. Additionally, the Coronavirus disease 2019 (COVID-19) pandemic began after our intervention was developed, with accompanying restrictions on physical activity and interaction due to social distancing. In future programs, it is necessary to develop exercise interventions that can be applied within the COVID-19 context.
Nevertheless, Intervention Mapping can serve as a blueprint for adapting the intervention to different populations and environs while retaining the core program components. It is possible to reorganize and utilize training tailored to the target audience before the application of ITAI. Furthermore, as a strategy to increase utilization in practice, it is necessary to establish a web-based system that allows the target person to be linked from the hospital to the community dementia prevention center for healthcare providers as a form of management. It can be used for patient treatment by establishing a system in which the patient management results are linked to the hospital for future use.
CONCLUSION
In conclusion, the Intervention Mapping was used as a methodical procedure for developing the ITAI for older adults with MCI. Healthcare providers can use this program to improve treatment adherence for chronic disease management and dementia prevention in older adults with cognitive impairment. Further studies are warranted to evaluate the effectiveness of this intervention. Efficacy assessment would contribute to strategies for improving treatment outcomes in older adults with MCI.
Notes
Authors' contribution
Study conceptualization and methodology - JS, EC, GSK, HK, BSY, and CGP; Data collection and analysis - JS and EC; Drafting and critical revision of the manuscript – JS, EC, GSK, HK, BSY, and CGP; Supervision - JS and EC; All authors have read and agreed to the published version of the manuscript.
Conflict of interest
No existing or potential conflict of interest relevant to this article was reported.
Funding
This research was supported by the 2020 Health Fellowship Foundation.
Data availability
All data generated or analyzed during this study are included in this published article.
Acknowledgements
This article is based on the doctoral dissertation of the first author Shin Jinhee from Yonsei University.
We acknowledge the clinic physicians and participants and caregivers of this study for their cooperation.