Effects of excretion care with a smart automatic defecation treatment system on skin humidity, incontinence-associated dermatitis, and pressure ulcers of patients with incontinence residing in long-term care facilities: Non-equivalent control group non-synchronized design
Article information
Abstract
Purpose
This study investigated the effects of excretion care using a smart automatic defecation treatment system on skin humidity, pressure ulcer (PU) occurrence, and severity of incontinence-associated dermatitis (IAD) among patients with incontinence residing in long-term care facilities.
Methods
Each patient in the experimental group was fitted with a smart automatic defecation treatment system. The system detected the release of urine/stool via a built-in sensor, followed by suction, cleaning with a water jet, and drying with warm air. Incontinence management for the control group involved the use of wet wipes for cleaning and changing diapers. The nursing interventions lasted for 2 weeks, and data were collected at baseline, week 1, and week 2. Descriptive statistics, t-tests, and the repeated-measures analysis of variance were used for data analysis.
Results
The analysis revealed statistically significant differences in skin humidity and IAD between the experimental and control groups.
Conclusion
This study is pertinent because it demonstrated the positive effects of excretion care using a smart automatic defecation treatment system on skin humidity, PU risk, and IAD among patients with incontinence residing in long-term care facilities. Further research is necessary to investigate additional factors such as pressure and physiological traits.
INTRODUCTION
1. Necessity of the Study
In South Korea, the average remaining life expectancy at 75 years of age is 13.3 years, which is higher than the overall average for Organization for Economic Co-operation and Development countries. These demographic changes have led to a growing number of older adults with chronic diseases who often experience urinary incontinence due to conditions such as dementia, stroke, and neurogenic bladder seeking long-term care facilities [1]. The prevalence of urinary incontinence announced in the United States is 15%~30% of those aged 65 years or older living at home, and the prevalence of fecal incontinence is 3%~15%. In the case of older adults residing in a nursing home, 65% of patients have both urinary and fecal incontinence [2].
Urinary incontinence has a significant effect on patients’ quality of life. Exacerbation of urinary incontinence is accompanied by a greater economic burden due to the costs of pads and the need to consider their waterproof design and absorption rate [3]. This ultimately leads to an increase in healthcare costs, making pressure ulcer (PU) prevention a crucial aspect of nursing care. PUs occur when continuous pressure is applied to deep tissues beneath intact skin, particularly among patients with prolonged immobility [4]. Incontinence caused by excretions such as urine or feces exposes the skin to chemical stimulation and excessive moisture, and when exposed to weak pressure or shear force, it reduces the durability of the skin, causing inflammation, redness, erosion, peeling, etc., promoting bedsores [5]. Severe PU can progress to life-threatening sepsis [6]. Therefore, it is crucial to assess the degree of skin damage of immobile patients and implement preventive measures to reduce the risk of PU [7].
PUs can be classified into the following six categories: (1) suspected deep tissue damage; (2) stage 1, with redness but without skin damage (the healing period is short and it is easily cured); (3) stage 2, with epidermal and partial-thickness skin damage that extends to part of the dermis; (4) stage 3, with full-thickness skin damage exposing the subcutaneous function (the fascia, muscle, and bone are not exposed); (5) stage 4, with full-thickness skin damage that exposes the fascia, muscle, bone, and supporting tissue; and (6) the last stage, which is unclassifiable. Nurses should assess the patient’s risk of developing PUs and provide preventive care, including frequent repositioning, management of excretions, and implementation of measures to prevent friction, to preserve the patient’s skin integrity [8].
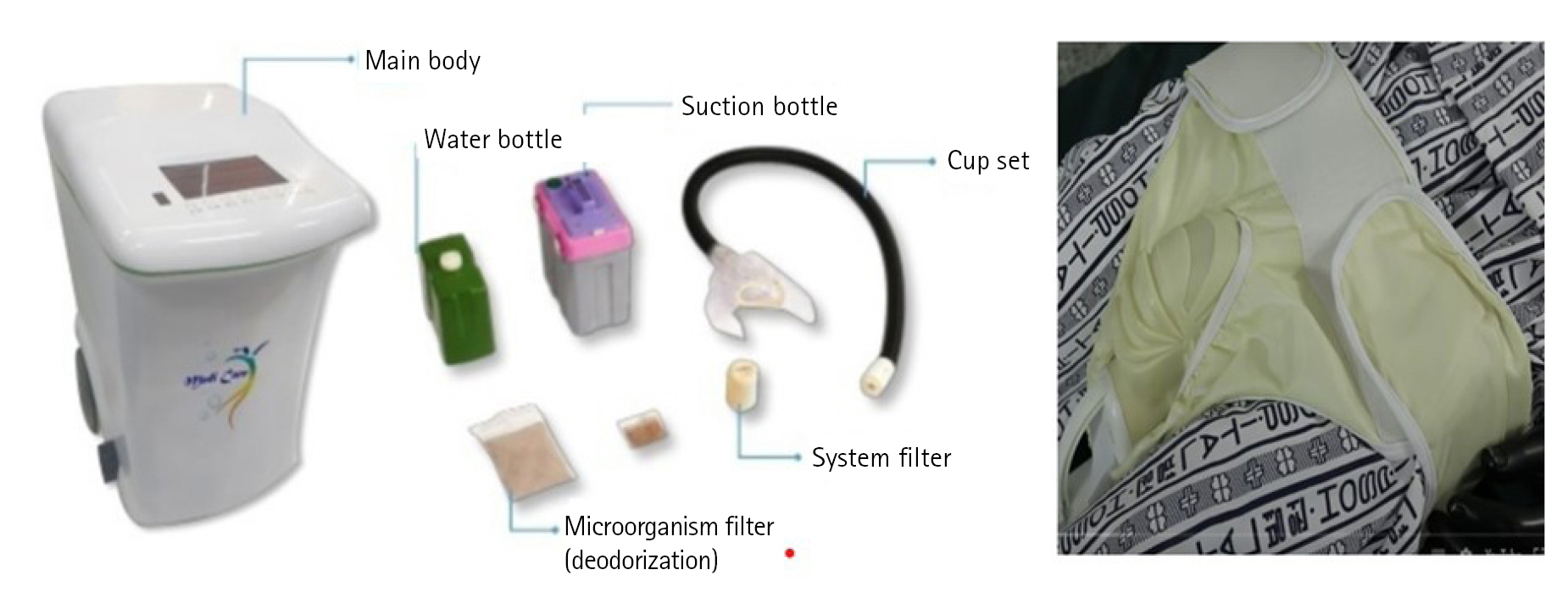
This study utilized a smart automatic defecation treatment system (Smart Body Clean by MEDIENVITECH Co., LTD.). The system resembles a diaper, with silicone material in direct contact with the skin, and is designed to be worn by patients with incontinence. Recent studies that investigated smart devices have demonstrated the utilization of robotic technology not only in the surgical, diagnostic, and therapeutic fields but also in the rehabilitation and nursing settings [9]. Another study demonstrated that the application of a silver-care robot program for individuals with impaired cognitive function delayed the onset of cognitive decline and even enhanced their performance of the activities of daily living [10]. Another study demonstrated the effects of using a bidet robot for defecation nursing on reducing the risks of incontinence-associated dermatitis (IAD) and PU, as well as lymphocyte levels, while improving serum albumin levels of critically ill patients [11]. However, few studies performed in South Korea have evaluated the effects of a smart automatic defecation treatment system on the risk factors of PU in long-term care facilities, where the population of patients with incontinence is the highest. Therefore, we aimed to address the gap in evidence and generate foundational data regarding the application of the smart automatic defecation treatment system, which is intended to reduce the risk of PU occurrence among patients with incontinence, while providing psychological care.
2. Purpose of the Study
The purpose of this study was to investigate the effects of a smart automatic defecation treatment system on the skin humidity, IAD, and occurrence of PU among patients with incontinence who are at risk for PU due to prolonged diaper use in long-term care facilities.
3. Research Hypotheses
Hypothesis 1: The experimental group that received defecation management applied with the smart automatic defecation treatment system will show a significant difference in skin humidity score over time compared to the control group.
Hypothesis 2: The experimental group that received defecation management applied with the smart automatic defecation treatment system will show a significant difference in IAD score over time compared to the control group.
Hypothesis 3: The experimental group that received defecation management applied with the smart automatic defecation treatment system will show a significant difference in occurrence of PU score over time compared to the control group.
METHODS
Ethical statement: This study was approved by the Institutional Review Board (IRB) of Jeonbuk Science College (IRB No. 2-7008126-A-N-01-232302-HR-01-01). Informed consent was obtained from the participants.
1. Study Design
This quasi-experimental study was conducted using a non-equivalent control group non-synchronized design to assess the effectiveness of a smart automatic defecation treatment system for patients with incontinence. This study was described according to the CONSORT 2010 checklist (https://www.equator-network.org/reporting-guidelines/consort/).
2. Participants
Participants were recruited through convenience sampling after identifying the long-term care facility in Gangwon Province with the highest number of patients with incontinence. The inclusion criteria were as follows: (1) patients capable of communication; (2) patients using diapers; (3) patients with impaired mobility and incontinence; and (4) patients who understood the purpose of the study and provided informed consent either personally or through their legal representatives. The exclusion criteria were as follows: (1) dementia; (2) unconsciousness; (3) use of a Foley catheter; (4) mental disorders; and (5) visual or hearing impairment.
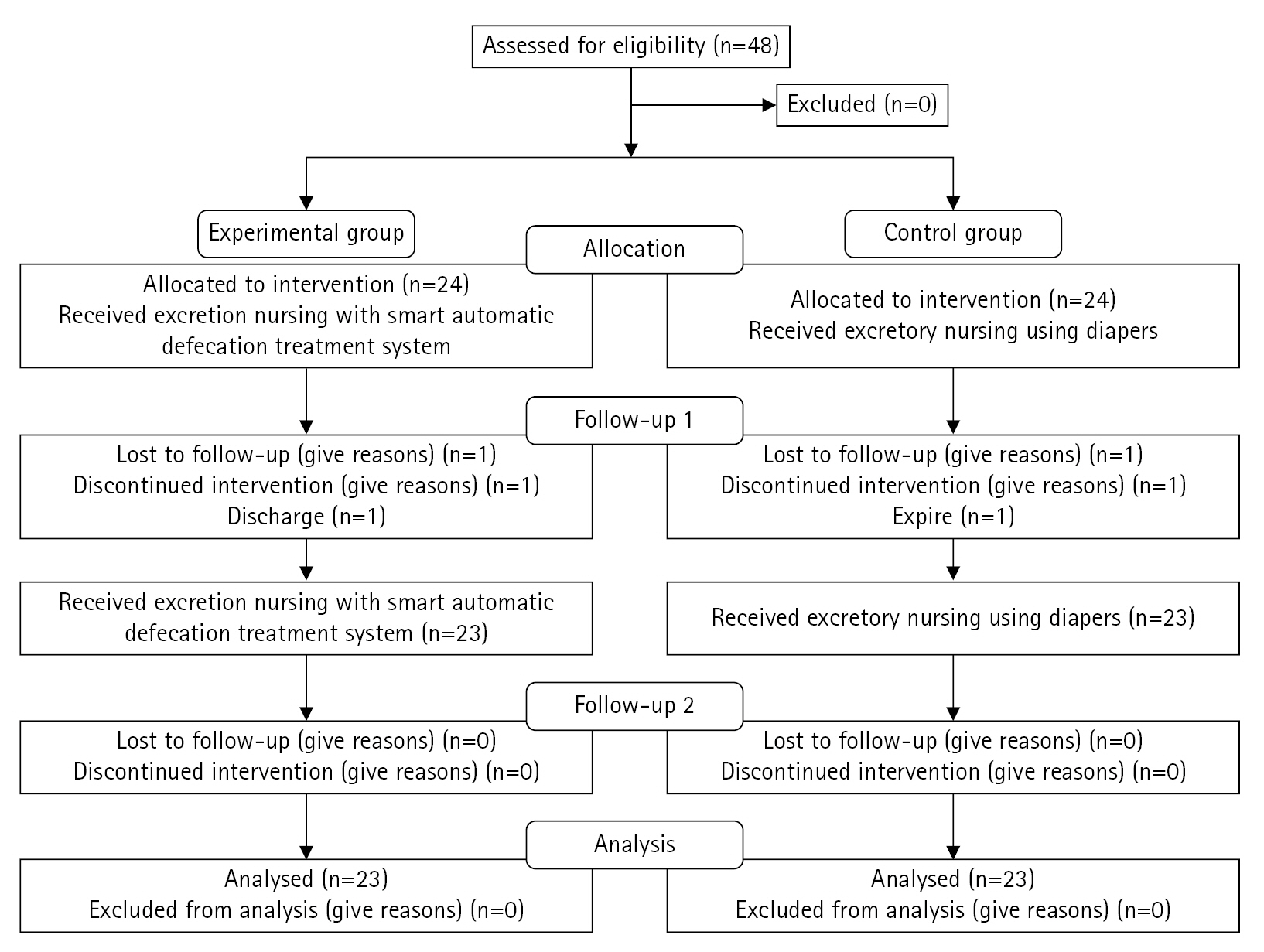
The sample size was calculated using G*Power 3.1 software, with statistical power (1-β) set at 0.75 for the repeated-measures analysis of variance (ANOVA), significance level (α) of 0.05 for two-tailed tests, and effect size (d) of 0.50. The minimum required sample size for each group was calculated to be 23. Therefore, considering the possibility of dropout, we initially selected a total of 48 patients, including 24 participants in each group. However, at the time of evaluation after 1 week, one person in the experimental group was discharged and one person in the control group died, so data from 46 participants were ultimately included. The smart automatic defecation treatment system was also applied to the control group upon completion of the experiment due to ethical considerations.
3. Application of the Smart Automatic Defecation Treatment System
1) Preparatory Training of the Researcher and Research Assistants
The researcher and research assistants underwent preparatory training prior to implementing the smart automated defecation treatment system for excretion care. A 3-hour training session during which three nurses from the long-term care facility in Gangwon Province (hereinafter referred to as the “study site”) were instructed about the research process, data collection methods, evaluation tools, evaluation methods, operation of the care robot, and application of the system to patients was conducted. A research assistant conducted the evaluation and data collection. The training session involved explanations, demonstrations, and hands-on practice, allowing the nurses to familiarize themselves with the procedures.
2) Application of the Smart Automatic Defecation Treatment System
First, based on evidence-based clinical nursing guidelines for PU care [12], the nurses were instructed to provide care to the buttocks and perineal area for all participants. The process included positional changes every 2 hours, 10 times per day, according to the guidelines for PU assessment, prevention, and management. Each patient in the experimental group was fitted with the smart automated defecation treatment system that provided excretion care. The 'Smart Automatic Defecation Treatment System (Smart Body Clean by MEDIENVITECH co., LTD.) is a product that has passed the usability evaluation and has obtained Korea Certification and Conformité Européene certifications in Korea, China Compulsory Certificate certification in the United States, and Federal Communications certification in China. A commercially available product is shown in Figure 1. The system automatically detects excreta via its built-in sensor and performs suction, followed by cleaning with a water jet and drying with warm air. The control group underwent the conventional method of cleaning with wet wipes and new diaper placement for 2 weeks. A research assistant directly installed the system for the experimental group; to prevent the halo effect, different researchers performed the interventions and observations. Both the experimental and control groups underwent measurements of skin humidity, severity of IAD, and stage of PU at baseline (pre-test). The observer directly measured these variables again at week 1 (post-test 1) and week 2 (post-test 2). After the study, the experimental group and the control group were provided with a given product.
4. Measurement Instruments
1) Participants’ General Characteristics
A structured questionnaire was used to collect information about participants’ sex, age, disease classification, stool pattern, and use of restraint belts.
2) Skin Humidity
Optimal skin humidity reduces the risk of skin damage caused by excessive humidity on the skin surface, such as incontinence-associated humidity [13]. Therefore, for participants wearing diapers, skin humidity was measured in three areas prone to PU occurrence (the perineal area, lower left buttock, and lower right buttock). Skin humidity was assessed using a 4-point Likert scale (1=very wet; 2=wet; 3=slightly wet; 4=dry). In clinical practice, the same measurement was made every day at 1 PM when bedsores and skin were checked. A research assistant consistently measured skin humidity by palpating the skin at baseline, week 1, and week 2 at 1:00 pm.
3) Incontinence-Associated Dermatitis and its Severity Instrument (IADS)
The symptom severity of IADS was measured using the IADS developed by Borchert et al. [14]. The IADS evaluates the following 13 anatomical regions: perineal skin; gluteal fold; lower left buttock; lower right buttock; left upper buttock; right upper buttock; genitalia (labium/scrotum); lower abdomen/suprapubic area; skin folds between genitalia and thighs; inner left thigh; inner right thigh; left posterior thigh; and right posterior thigh. Symptom severity is rated using a 5-point Likert scale: 0=no erythema; 1=pink erythema; 2=red erythema; 3=inflamed skin (with irregular borders and small red spots extending from the edges); and 4=skin loss. Higher scores indicate more severe IADS. In this study, the mean score was calculated for evaluation purposes. In a development study by Borchert et al., Cronbach's α value was .98. The instrument demonstrated good reliability in this study, with Cronbach’s α of .86.
4) Stages of PU
The stages of PU development were determined using the European Pressure Ulcer Advisory Panel classification system [15]. This system classifies PU into four stages based on the severity of skin damage: stage 1=intact skin with non-blanchable erythema; stage 2=partial-thickness loss of dermis; stage 3=full-thickness tissue loss; and stage 4=full-thickness tissue loss with exposed bone, tendon, or muscle. In this study, the instrument demonstrated good reliability, with Cronbach’s α of .92.
5. Data Collection
Data were collected from February 15 to May 20, 2023. The study protocol was reviewed by the responsible department at the study center, and permission was granted by the director. Skin humidity, severity of IAD, and PU stages were assessed as risk factors for PU occurrence among patients with fecal incontinence, and the results were utilized as preliminary survey data for this study. Based on the participants’ general characteristics and risk factors for the PU stages, they were assigned to the experimental or control group according to the order generated by a random number table. Two post-tests were conducted at weeks 1 and 2 to assess skin humidity, PU stage, and IAD severity. Participants were assured that all collected data would be immediately discarded after research presentation. They were also informed that they could withdraw consent to participate at any time if they or their caregivers decided to discontinue their involvement. Figure 2 presents the flowchart of the study process based on the 2010 CONSORT flow diagram template.
6. Data Analysis
Data analysis was performed using SPSS/WIN 23.0 software (IBM Corp.). Descriptive statistics were used to analyze the participants’ socio-demographic and disease-related characteristics. The homogeneity of the experimental and control groups was verified using the Shapiro-Wilk and Mann-Whitney U-tests. The repeated-measures ANOVA was used to assess the effects of the experimental intervention.
7. Ethical Considerations
Prior to data collection, ethical protection of the participants was ensured by obtaining approval from the IRB of the Jeonbuk Science College Ethics Committee (IRB no. 2-7008126-A-N-01-232302-HR-01-01). Participants were recruited via announcements posted on the bulletin board by the personnel in charge. Potential participants and their caregivers who expressed willingness to participate in the study were provided explanations about the purpose and procedure of the experiment and access to medical records. Among the patients who voluntarily provided written informed consent, those who met the inclusion criteria were enrolled in the study.
RESULTS
1. Characteristics and Homogeneity Test of Experimental and Control Groups
A total of 46 patients participated in the study (23 each in the experimental and control groups). The mean ages of the experimental and control groups were 79.48±8.94 years and 80.09±10.37 years, respectively. All participants were women with an overall mean age of 79.79±9.66 years (range: 56~97 years). The general characteristics did not differ significantly between the two groups. No statistically significant intergroup differences were observed with respect to cardiovascular diseases, Parkinson’s disease, cancer diagnoses, and stool patterns (Table 1).
2. Homogeneity Testing of the Pre-Intervention Dependent Variables Between the Experimental and Control Groups
First, the Shapiro-Wilk test was conducted to test the intergroup homogeneity of the dependent variables, namely, skin humidity, IAD severity, and PU occurrence. Due to the small size of the control group (≤50) and violation of the normality assumption, the Mann-Whitney U-test was used to test for homogeneity. The results revealed no statistically significant differences in the dependent variables between the experimental and control groups. Therefore, it can be assumed that the two groups were statistically similar based on the established intergroup homogeneity of the baseline dependent variables (Table 2).
3 Hypothesis Testing
1) Hypothesis 1
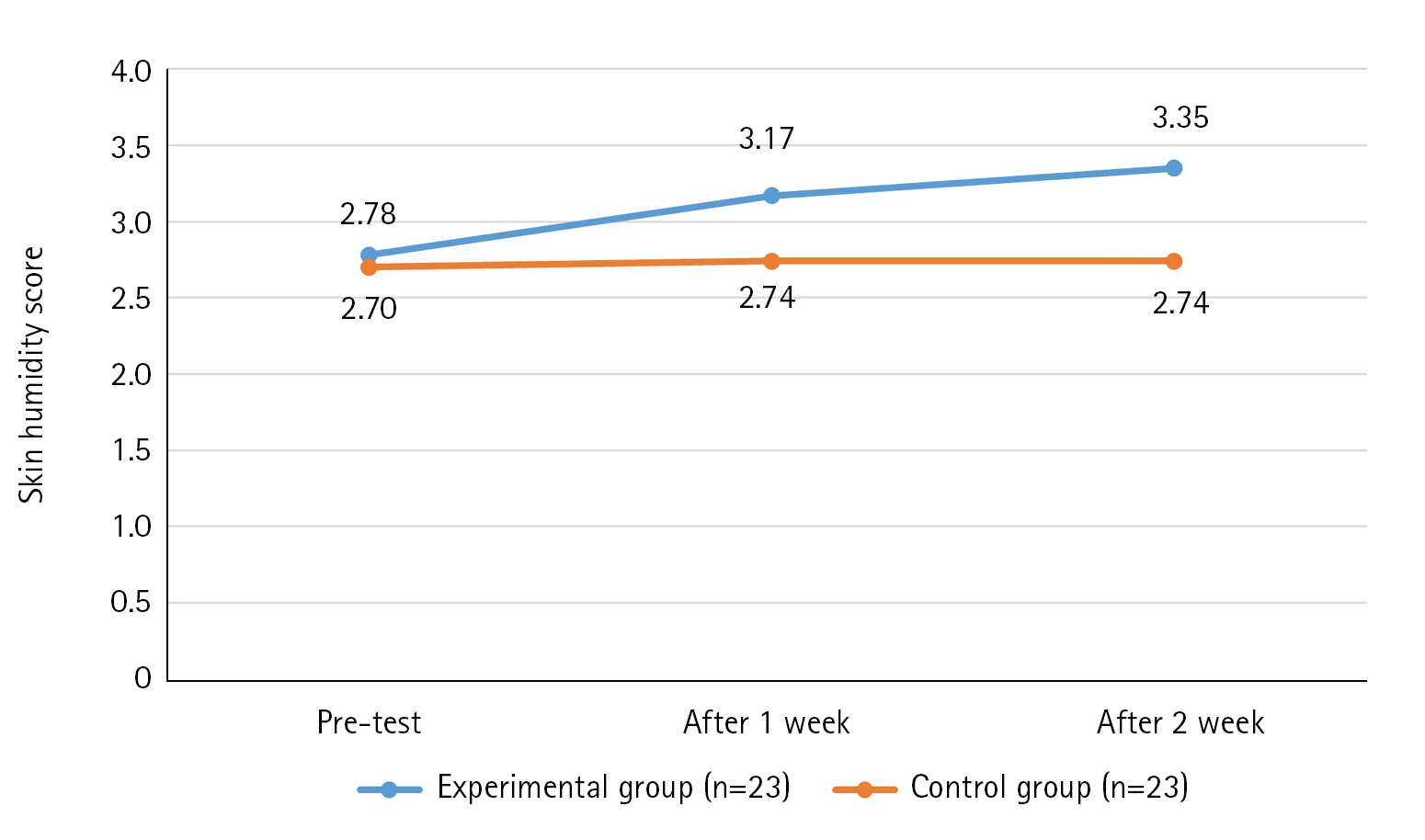
The results of the skin humidity score over time of the experimental and control groups showed that the variance structure between the groups was equal, satisfying the sphericity assumption (p=.978). Additionally, statistical significance was established for intergroup difference (F=22.95, p<.001), changes over time (F=5.30, p=.007), and the interaction between time and group (F=3.80, p=.026). Thus, the first hypothesis was supported (Table 3, Figure 3).

Effect of Defecation Nursing Using a Smart Automatic Defecation Treatment System on Skin Humidity (N=46)
2) Hypothesis 2
The results of the IAD score over time of the experimental group and control group showed that the variance structure between the groups was equal, satisfying the sphericity assumption (p=.865). Additionally, statistical significance was established for the intergroup difference (F=17.18, p<.001), changes over time (F=3.51, p=.034), and the interaction between time and group (F=4.81, p=.010). Thus, the second hypothesis was supported (Table 4, Figure 4).

Effects of Defecation Nursing Using a Smart Automatic Defecation Treatment System on Incontinence-Associated Dermatitis (N=46)
3) Hypothesis 3
The results of the PU score over time of the experimental group and control group showed that the variance structure between the groups was equal, satisfying the sphericity assumption (p=.966). However, although the intergroup difference was statistically significant (F=4.15, p=.048), changes over time (F=1.07, p=.351) and group×time interaction (F=2.58, p=.087) did not attain statistical significance. Thus, the third hypothesis was rejected (Table 5, Figure 5).

Effect of Defecation Nursing Using a smart Automatic Defecation Treatment System on the of Pressure Ulcer (N=46)
DISCUSSION
Considering that patients with wet skin conditions caused by urinary or fecal discharge have a 2.4-fold higher risk of PU occurrence compared to those with dry skin, reducing humidity is essential for PU prevention [16]. Although humidity alone does not necessarily cause skin damage, it can easily lead to skin damage in the presence of irritants such as urine and feces. Therefore, eliminating irritants and reducing skin humidity are effective methods for preventing PU [17].
In this study, the significant decrease in skin humidity in the experimental group is believed to be the result of the automated defecation processing system quickly detecting and inhaling secretions such as urine or feces, washing the affected area with a water jet, and then drying it. It is believed that clean wind through an antibacterial microfilter is effective in controlling the subject's skin humidity. However, there is a limitation in that an objective device for measuring humidity was not used.
In this study, we found a significant decrease in IAD in the experimental group, which means that the sensing system and bidet function are effective in reducing IAD by removing irritation and keeping the skin clean. Among the preceding studies, both the fecal management using bidet robots [11] and the study on fecal management using urine and stool detection sensors [18] were significant, consistent with the results of this study. This has confirmed that immediate treatment, cleaning, and drying of excrement is effective in reducing IAD. These results confirmed that proper cleansing of the perineum and the use of protective agents are essential for protecting the perineum skin, reducing IAD, and reducing the risk of PU [19].
In this study, there was a difference in the frequency of PUs between the experimental and control groups. This difference may be attributed to the irritant removal and humidity control performed by the smart automatic defecation treatment system utilized in our study. The group×time interaction was not statistically significant. This is thought to be because PU are affected by pressure (friction and shear forces) [20], malnutrition, old age, anemia, sensory loss, mobility, confusion, hemoglobin and serum albumin levels [21] as well as water. This study has limitations due to the intervention of suction, cleaning, and drying functions. A repeat study considering other variables is suggested.
The results of the study using bidets [11] were significant in group×time interaction and between groups, which was different from the results of this study. The reason could be that in the previous study, the subjects were young and physiological variables were also taken into consideration. In a study [18] on the prevention of bedsores using diaper sensors in older adults, the results were found to be not significant. The results of this study showed that the group×time interaction was not statistically significant, but between groups was significant. This is believed to be the result of the short period and failure to consider various variables such as the subject's age, physiological variables, and pressure.
CONCLUSION
This study demonstrated the positive effects of utilizing a smart automatic defecation treatment system as excretion care for residents of long-term care facilities. The findings indicated improvements in skin humidity and alleviation of IAD, thereby supporting its use as a nursing intervention for PU prevention. However, although there was a difference in the occurrence of PU between groups, it was not found to be significant in group x time, showing that it had no effect. Future research should explore the application of the smart automatic defecation treatment system among community-dwelling older adults. It is also important for future studies to consider various variables associated with PU occurrence and use objective tools with high sensitivity for verification.
Notes
Authors' contribution
Study conception and design acquisition - EJK and EYK; Data collection - EJK; Analysis and interpretation of the data - EYK; Drafting and critical revision of the manuscript - EJK and EYK; Final approval - EJK and EYK
Conflict of interest
No existing or potential conflict of interest relevant to this article was reported.
Funding
This research was funded by MediEnvi-Tech, Co., Ltd., Wonju, South Korea.
Data availability
Please contact the corresponding author for data availability.
Acknowledgements
None.